(This is the English version of the mini-presentation we presented at the 10th Panhellenic Congress of the Hellenic Academy of Neuroimmunology in Thessaloniki, Greece, held on 14-17 December 2024. Also, available on my LinkedIn: https://www.linkedin.com/pulse/how-ai-can-combat-alzheimers-emmanouil-dimarellis-ug4vf)
In Neurology, Artificial Intelligence isn’t just an option; it’s a game-changer. Every Neuroscientist must grasp the potential of this technology to stay ahead of the curve and avoid lagging behind both colleagues and competition. Let’s have a brief look at what it can do, and how it can be used effectively to help you in your research.
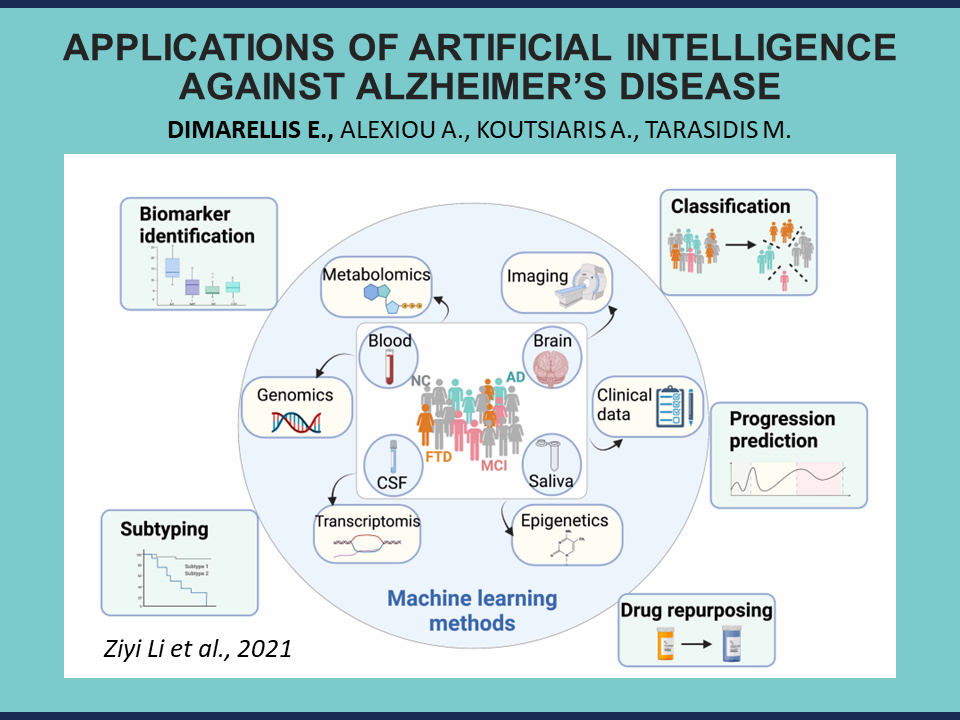
Everything begins with the comprehension of the disease that we are trying to cure.

Disease comprehension means that we understand the specific mechanisms that drive the human body to weaken. It means that we identify biomarkers and biochemical pathways that are important for the disease, and we uncover new therapeutic targets or ways of intervention. Without this important step, we cannot develop better diagnostics or therapeutics, and we cannot determine optimal healthcare practices.
Machine learning techniques, such as Principal Component Analysis, and bioinformatics methods, such as Differential Expression Analysis, are commonly employed to identify the most significant genes among all possible ones. As mentioned above, comprehension comes first, so these methods are usually used at the beginning of each bioinformatics analysis.
Next, we have Artificial Intelligence applications for Alzheimer’s diagnosis.

In this slide, we see the workflows of two really interesting analyses. The key to note here is that AI can utilize datasets of various types of data, such as Whole Blood RNA Gene Expressions and MRI images. A Machine Learning model can find relationships between data points and groups of individuals, so subtle that the human brain would need ages to uncover them. This happens in two phases: the training phase and the testing phase.
At the training phase, we feed the model a dataset, called the training dataset, which contains the values of (for example) the expression levels of some genes, and other characteristics of each sample (i.e. if these values are from a patient or control). The model is programmed in a way that enables it to make predictions based on the expression levels, but at this phase, its predictions are somewhat random. Every time it makes a prediction, it checks whether it is right or not and it tunes itself to make a better prediction the next time. If the model is good and is trained on sufficient data, then it will be able to make accurate predictions at the end of the training phase.
This is what we test at the testing phase; How accurate is our model? We check this by feeding the model a new dataset, which is called the testing dataset, and now the model makes all predictions without tuning itself anymore. This process produces various metrics that show how good is the model. If it is worth it, it may be used in real-world problems and diagnose for example a patient that has Alzheimer’s disease, ideally without the invasive lumbar puncture.
Next, we have Artificial Intelligence applications for Alzheimer’s prognosis.

The difference between diagnosis and prognosis is that prognosis is used to predict if a younger individual without any symptoms will develop a disease in the future. It is different from diagnosing somebody who already has the disease. We hope to make this difference clear with the pictures on the slide.
As you can see (okay, zoom a bit :’D), Sam the doctor and Nick the individual are young in the first picture, but they get older in the 3rd and 4th pictures. Sam collected blood samples from Nick two different times (or even more) and analysed the blood samples he collected longitudinally. He did so because he wanted to check how the gene expressions changed over time. Of course, Nick was not the only individual that gave samples. Many individuals such as Nick gave samples, and Sam managed to identify key gene expression changes over time that were statistically and significantly different between those who in the end developed the disease and those who didn’t.
Until today, the models that approach this schema are only partly prognostic, because they are based on neuropathological findings from longitudinal MRI scans and focus more on an early diagnosis rather than prognosis. Early diagnosis means that the individual has already shown pathophysiological changes but hasn’t had the clinical symptoms of the disease. This classification is important because when these pathophysiological changes have occurred, it may not be possible to convert the situation, whereas a prognosis allows for an early treatment that might prevent the development of the disease overall. The UK BioBank initiative aspires to produce the data needed for real prognostic approaches.
Next, we have Artificial Intelligence applications for Drug Repurposing for AD.

One slide and some paragraphs cannot describe our enthusiasm for this AI application! Essentially, many drugs have passed the first clinical trial and are considered safe but their therapeutic potential is not known yet. Perhaps, they got developed for a specific disease but they didn’t achieve the expected or desired results. However, the fact that a drug has not worked for a specific disease does not imply that the drug lacks therapeutic potential for any disease or that it cannot achieve the desired results when combined with the right mix of drugs.
In this way, many drugs exist that, while we know that they are safe, we do not use them because we don’t know how to use them and to which disease. The number of different combinations of drugs is enormous! This is where AI comes in handy. AI can find which drugs are similar to drugs that we already use for a disease from a big dataset to speed up the drug selection process and allow us to start in vitro and in vivo experiments on these drugs. This is so helpful for two reasons: a) cost efficiency and b) time efficiency. We only test the most probable drugs, saving time and money. If we take into account the cost we avoid as these drugs have already been tested for safety measures, this process is invaluable!
We suggest that you go and read the whole paper of (Chenglong X. et al., 2022) to get the bigger picture of a complete research article that includes both in-silico analysis and wet-lab experiments.
Finally, we have AI for experimental model selection.

For this application, we could not provide an example referring to Alzheimer’s Disease, because this idea is new and still under development. The ideal scenario would be to have a PC simulator, put the drug and the experimental model (e.g HeLa cells) and see the simulated results. If the results were good, further experimentation would be considered, as opposed to bad results. The good news is that this idea is partially achieved, at least toxicologically speaking. The RASAR initiative helps identify if a drug is toxic for a certain model, so a quick check may prevent many €€ from being burnt in the wrong model. However, a broader simulation, the aspiration of the IMPROVER initiative, is not yet available due to insufficient data.
That is all we have for you today. Thank you for your attention!
References
Aksu, Y., Miller, D. J., Kesidis, G., Bigler, D. C., & Yang, Q. X. (2011). An MRI-Derived definition of MCI-To-AD conversion for Long-Term, automatic prognosis of MCI patients. PLoS ONE, 6(10). https://doi.org/10.1371/journal.pone.0025074
Kononikhin, A. S., Zakharova, N. v., Semenov, S. D., Bugrova, A. E., Brzhozovskiy, A. G., Indeykina, M. I., Fedorova, Y. B., Kolykhalov, I. v., Strelnikova, P. A., Ikonnikova, A. Y., Gryadunov, D. A., Gavrilova, S. I., & Nikolaev, E. N. (2022). Prognosis of Alzheimer’s Disease Using Quantitative Mass Spectrometry of Human Blood Plasma Proteins and Machine Learning. International Journal of Molecular Sciences, 23(14). https://doi.org/10.3390/ijms23147907
Li, Ziyi, Jiang, X., Wang, Y., & Kim, Y. (2021). Applied machine learning in Alzheimer’s disease research: Omics, imaging, and clinical data. In Emerging Topics in Life Sciences (Vol. 5, Issue 6, pp. 765–777). Portland Press Ltd. https://doi.org/10.1042/ETLS20210249
Luechtefeld, T., Marsh, D., Rowlands, C., & Hartung, T. (2018). Machine learning of toxicological big data enables read-across structure activity relationships (RASAR) outperforming animal test reproducibility. Toxicological Sciences, 165(1), 198–212. https://doi.org/10.1093/toxsci/kfy152
Marzi, S. J., Schilder, B. M., Nott, A., Frigerio, C. S., Willaime-Morawek, S., Bucholc, M., Hanger, D. P., James, C., Lewis, P. A., Lourida, I., Noble, W., Rodriguez-Algarra, F., Sharif, J. A., Tsalenchuk, M., Winchester, L. M., Yaman, Ü., Yao, Z., Ranson, J. M., & Llewellyn, D. J. (2023). Artificial intelligence for neurodegenerative experimental models. In Alzheimer’s and Dementia. John Wiley and Sons Inc. https://doi.org/10.1002/alz.13479
Montana, G., & Payan, A. (2015). Predicting Alzheimer’s disease: a neuroimaging study with 3D convolutional neural networks. http://adni.loni.usc.edu/wp-content/uploads/how
Nanni, L., Interlenghi, M., Brahnam, S., Salvatore, C., Papa, S., Nemni, R., & Castiglioni, I. (2020). Comparison of Transfer Learning and Conventional Machine Learning Applied to Structural Brain MRI for the Early Diagnosis and Prognosis of Alzheimer’s Disease. Frontiers in Neurology, 11. https://doi.org/10.3389/fneur.2020.576194
Xie, C., Zhuang, X. X., Niu, Z., Ai, R., Lautrup, S., Zheng, S., Jiang, Y., Han, R., Gupta, T. sen, Cao, S., Lagartos-Donate, M. J., Cai, C. Z., Xie, L. M., Caponio, D., Wang, W. W., Schmauck-Medina, T., Zhang, J., Wang, H. ling, Lou, G., … Fang, E. F. (2022). Amelioration of Alzheimer’s disease pathology by mitophagy inducers identified via machine learning and a cross-species workflow. Nature Biomedical Engineering, 6(1), 76–93. https://doi.org/10.1038/s41551-021-00819-5
Zhang, Y., Miao, Y., Tan, J., Chen, F., Lei, P., & Zhang, Q. (2023). Identification of mitochondrial related signature associated with immune microenvironment in Alzheimer’s disease. Journal of Translational Medicine, 21(1). https://doi.org/10.1186/s12967-023-04254-9
~~~~~ For you, Eagle-eyed Investigator, here is a little secret: AI can help you make incredible images for your slides just like I did to help you with your presentation 😉 ~~~~~